Technology - Colors
Inorganic compounds, such as ruby (Al2O3: Cr) are colored in red color that originally Al3 + part of colorless alumina Al2O3 is replaced by Cr3 +. Chromium oxide Cr2O3 is known it is well to show the green but, Al3 + part of in the alumina crystal shows the red and replaced by Cr3 +. This is one of the very strange phenomenon.
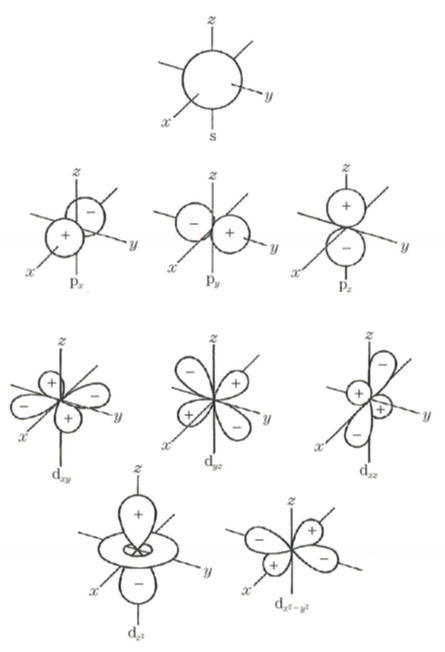
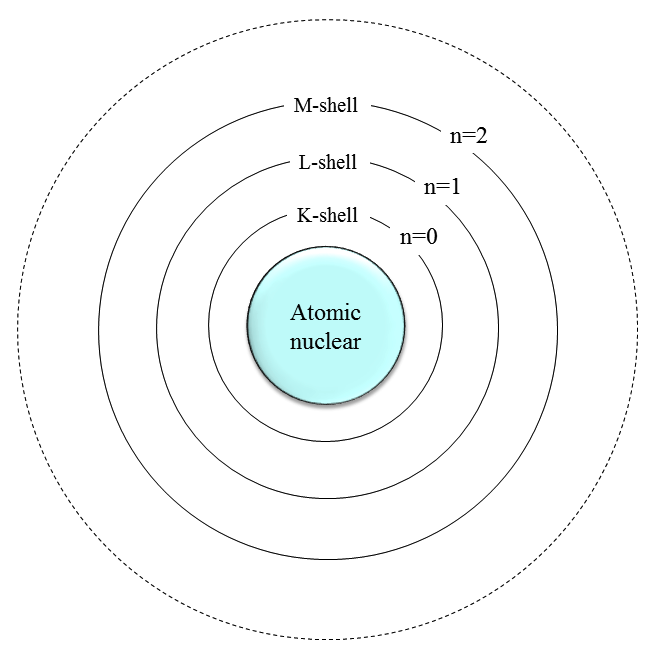
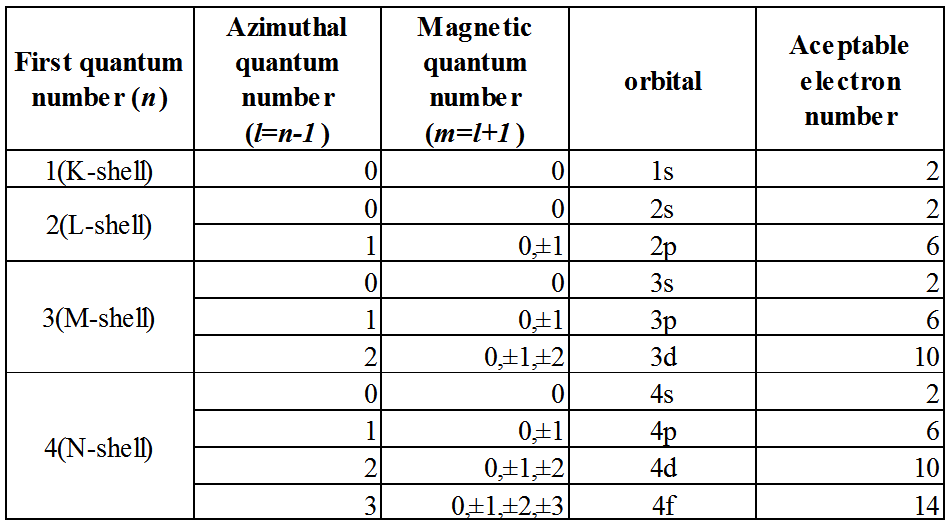
Atom consists of nucleus and electron consisting of protons and neutrons. The electrons are present so as to draw a trajectory around the atomic nucleus, we call the orbit this electrons exist as electron orbit. K around the atomic nucleus, L, there is a nucleus M (corresponding to the principal quantum number), we have s, p, the deputy shell trajectory such as d further depending on each orientation quantum number. (For details, see Chart)
When making a molecule with atoms bonded, because sharing electrons with each other in close proximity are atoms forming the molecule (ionic bond or covalent bond), and complicates each electron orbit overlap. Especially in split orbit of the outermost shell is affected to form a new orbit. This called the ligand field splitting. The interaction between the atoms, atoms around not only the neighboring atoms, especially affects large atomic size.
Transition metals that are frequently used in the coloring material of inorganic has a trajectory of large M shell of size, the d orbitals are not completely filled with electrons. When the transition metal atom is bonded to an oxygen complex electronic orbits the ligand field described above is formed, the track in the M shell has the energy level that corresponds. Electrons to be energetically stable exist in various orbital (ground state). When it is irradiated with an electromagnetic wave, the electron ground state will jump to the trajectory of the high energy level from the ground state by receiving the energy of the electromagnetic waves (called excited state). This excited state it will return to the ground state because it is extremely unstable.
Atomic structure described by Bohr's model
Configuration of s-, p-, d-orbitals